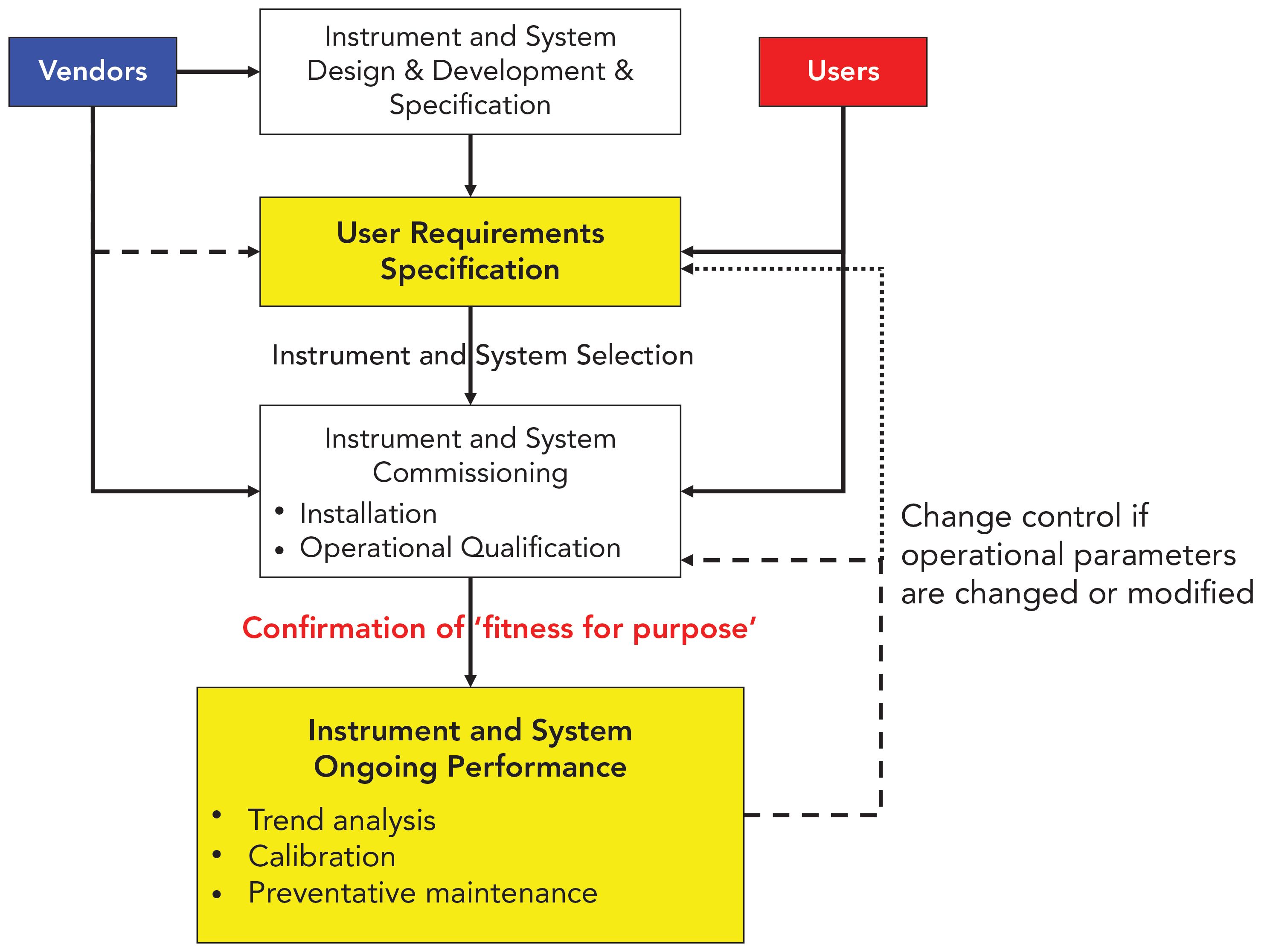
Analytical instrument qualification in capillary electrophoresis - Cianciulli - 2012 - ELECTROPHORESIS - Wiley Online Library

Qualification of analytical instruments for use in the pharmaceutical industry: A scientific approach | Semantic Scholar

AIQ Analytical Instrument Qualification. AIQ – Analytical Instrument Qualification Varian, Inc.'s Analytical Instrument Qualification documentation has. - ppt download
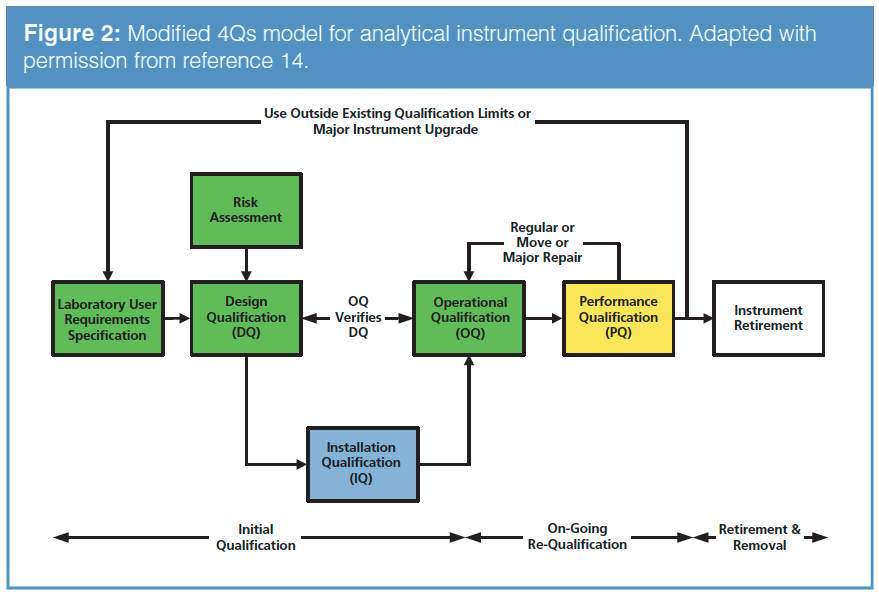
Qualifying Analytical Instruments: General Chapter <1058> Clarifies Terminology, Classifies Instruments

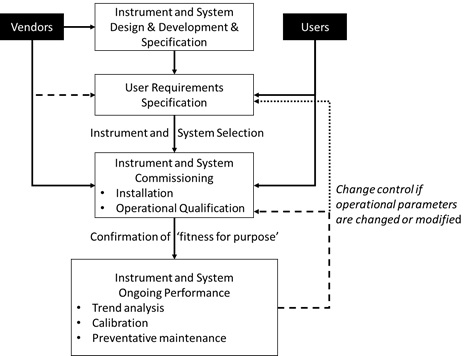

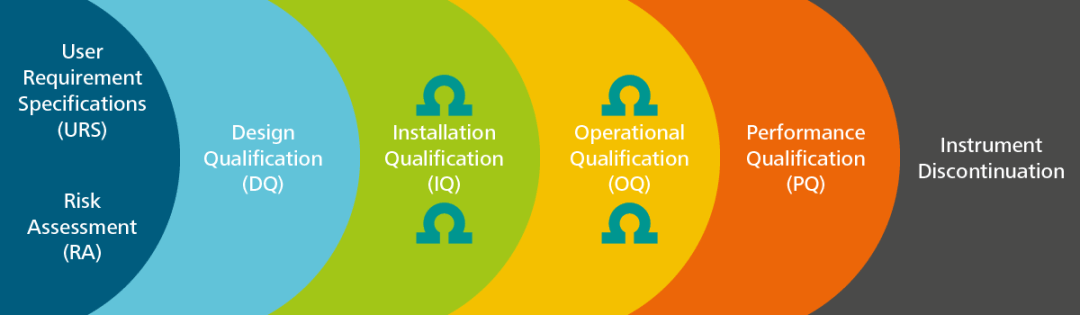




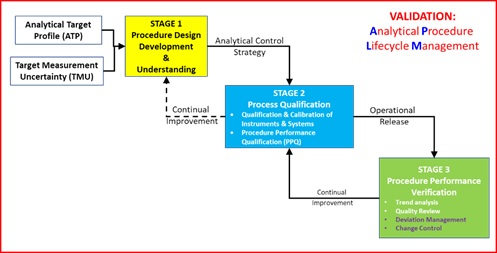




![PDF] á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION | Semantic Scholar PDF] á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/80ff4ccd1aa444bdff6ff42b1127686804f1bd40/2-Figure1-1.png)